Cartilage damage in the knee joint
Every year around 6 million patients worldwide are affected by knee cartilage damage – in Germany around 30,000 per year. [1]
Common causes of cartilage defects: brief, intense abnormal biomechanical loading or twisting of the knee joint in accidents and sports injuries, or as a result of excess biomechanical stress. Fragments of cartilage may come loose from the joint surface; these may vary in size depending on the severity of the damage.
In addition to traumatic defects, the bone condition osteochondrosis dissecans can also lead to damage to the protective cartilage. Likewise, axis misalignments may affect certain areas of cartilage as a result of biomechanical stress.
Limited regenerative capacity
Joint cartilage has no direct nerve or vascular supply: the highly specialised chondrocytes (cartilage cells) are largely “walled in” inside the cartilage's compact extracellular matrix (part of the tissue between the cells that surrounds them like a mesh).
After an injury, the joint cartilage has limited healing capacity due to the lack of an intrinsic regenerative response (the ability to recover under its own initiative). As a result, either no restoration occurs, or the damaged area is filled with biomechanically inferior replacement tissue.
M-ACI procedure
Matrix-associated autologous chondrocyte implantation for cartilage defects
M-ACI is used to compensate the lack of intrinsic regeneration – using the body’s own cells. This triggers the most complete healing of the cartilage damage possible: the implanted cartilage cells gradually repair the defect with biomechanically high-quality cartilage tissue.
With our smart biomaterials specially developed for cartilage cell implantation, it is possible to quickly and easily introduce the cultured cartilage cells into the defect.
Advantages of M-ACI
Matrix-associated autologous chondrocyte implantation has significant advantages over conventional therapies:
- Suitable for chondral and osteochondral cartilage defects
- Natural healing from within
- Long-term results demonstrated by evidence studies
- Avoidance of rejection reactions due to using the body’s own cells
- Small (up to 2 cm2) and large defects can be treated
- High product safety through strict quality requirements
- Strictly monitored cell proliferation and manufacturing under cleanroom conditions
- Implantation of a defined number of vital cells
- Standardised biopsy by certified specialists
| ACI has been recommended by the German Society for Orthopaedics and Trauma Surgery (DGOU) as a standard treatment for cartilage defects since 2004. The 2022 recommendations refer to treatments of small defects up to 2 cm2 in size.[2] |
|---|
How M-ACI works
Matrix-associated autologous chondrocyte implantation in three steps
The MACI procedure essentially consists of biopsy, processing, and implantation back into the defect area. The first and last step both involve minimally invasive surgery.
Procedure
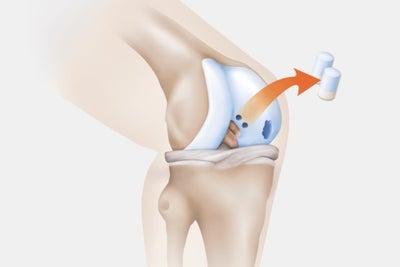
Step 1: Biopsy
- Taking a sample of blood and a small amount of cartilage (cartilage-bone punch) from a non-weight-bearing part of the healthy cartilage.
- Transport to our specialised production area – in special transport containers, provided free of charge.
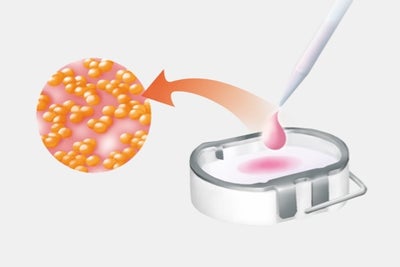
Step 2: Cell proliferation
- Isolation and culture of cartilage cells using procedures developed by TETEC AG.
- Introduction of the cultured cells into a carrier medium, e.g. biphasic collagen or albumin-hyaluronic acid matrix.
- Production of the personalised cell graft is completed approximately 3 weeks after biopsy.
- Delivery of our product to the treating doctor after extensive quality controls.
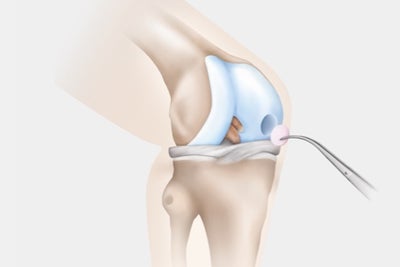
Step 3: Implantation
- Hospitalisation for a surgery of approximately 45 minutes:
- Clearing of damaged cartilage from the defect area
- Insertion of the graft to fit defect shape (matrix-supported or gel-based matrix-associated chondrocyte implantation).
- Synthesis of new cartilage tissue by the implanted cells.
- Complete maturation of the new tissue after approximately 12 months.
Cell biology
Cultivation of cartilage cells
To treat a cartilage defect with M-ACI, the implanted cells must be capable of producing new, high-quality cartilage tissue. However, the cartilage-specific properties of the collected cartilage cells may be lost when cultured. This process, known as dedifferentiation, is reversed by introducing cartilage cells into our biomaterials.
In order to check the quality of our individually produced cartilage cell products, we carry out cell and molecular biology quality controls for each product using validated methods. Using this analysis, we ensure that only vital and functional cell products are delivered.
Scientifically proven quality
An independent study[3], [4] of M-ACI variants available in Europe demonstrated the excellent quality of our biphasic collagen matrix: our products for matrix-assisted chondrocyte implantation consists predominantly of high-quality cartilage cells. Similarly convincing properties have also been demonstrated for our in-situ crosslinking hydrogel (products for gel-based matrix-associated chondrocyte implantation) be proven.
Research results from our development team have also been awarded prestigious scientific prizes, for example by the Osteoarthritis Research Society International (OARSI), the Association for Orthopaedic Research (AFOR) or the German Society for Orthopaedics and Trauma Surgery (DGOU).
Clinical outcomes
Over 90% success rate for knee cartilage defects
The results of long-term studies[5] and prospective randomised studies show: ACI is significantly superior to other methods of treating cartilage defects exceeding 4 cm2 in size. Success rates of over 90% were reported in studies over a period of 14 years.
Patients with limited degenerative cartilage damage can also benefit from ACI: Initial clinical studies also show good outcomes in such cases.
Other treatment methods
Therapy options for cartilage damage
Refixation
Broken-out cartilage-bone fragments can be reattached in fresh injuries: refixation of detached pieces is done using small pins that gradually dissolve. Depending on the location and size of the defect, this procedure is performed by arthroscopic surgery or via a small opening of the joint
Microfracture surgery
In the case of minor damage, the bone lying under the cartilage defect is broken open with small drills or chisels. Through the opening, blood and cells of the bone marrow can enter the defect. This results in biomechanically inferior scar tissue: the so-called fibrocartilage is significantly less resilient than the native (original) articular cartilage.
Osteochondral Autologous Transplantation (OATS)
In this procedure, cartilage-bone punch are taken from less stressed areas and inserted into the defect area. A large part of the damage can thus be refilled with articular cartilage. Less load-bearing fibrocartilage is formed in the spaces between the cylinders. The treatment is limited to smaller defects, since the use of too many cartilage-bone cylinders can lead to problems at the removal and recipient sites with the development of premature arthrosis.
Contact our sales and customer service
Our trained employees are pleased to support and advise you and your team. We are here for you if you have any questions about our products and services.
| Glossar | Explanation | Abbreviation |
| ACI | Autologous chondrocyte implantation, also known as autologous chondrocyte implantation or autologous cartilage cell implantation. This procedure is used to treat cartilage damage. Autologous cartilage cells are collected, cultured in a nutrient solution and inserted into the patient’s cartilage defect in order to reduce the discomfort caused by the cartilage damage and prevent the development of osteoarthrosis. | ACI |
| Matrix-associated ACI | Also commonly referred to as: matrix-coupled, matrix-supported or matrix-induced autologous chondrocyte implantation (M-ACI). In the M-ACI procedure, the cultivated autologous cartilage cells are implanted into the defective cartilage area using a carrier medium, such as a collagen matrix or a hydrogel. | MACI ACI-M |
| Autologous | From the same body | |
| Chondrozyten | Cartilage cells | |
| in-situ | Retention (of implanted cells) in the desired position in the body |
[1] https://www.qkg-ev.de/ueber-uns/was-ist-der-qkg/
[2] https://pubmed.ncbi.nlm.nih.gov/35189656
[3] Nuernberger et. al., The influence of scaffold architecture on chondrocyte distribution and behavior in matrix-associated chondrocyte transplantation graft.Biomaterials. 2011.
[4] Bretschneider et. al., Characterization of primary chondrocytes harvested from hips with femoroacetabular impingement.Osteoarthritis Cartilage. 2016.
[5] Angele et. al., Cell-based treatment options facilitate regeneration of cartilage, ligaments and meniscus in demanding conditions of the knee by a whole joint approach. Knee Surg Sports Traumatol Arthrosc. 2021.